18-2 Review and Reinforcement Determining the Strengths of Acids and Bases Answers
Acid and Base of operations Force
- Page ID
- 1314
All acids and bases do non ionize or dissociate to the same extent. This leads to the statement that acids and bases are not all of equal strength in producing H+ and OH- ions in solution. The terms "strong" and "weak" give an indication of the forcefulness of an acid or base of operations. The terms strong and weak describe the power of acid and base of operations solutions to behave electricity. If the acrid or base conducts electricity strongly, it is a potent acrid or base. If the acrid or base conducts electricity weakly, it is a weak acid or base.
Demonstration of Acrid and Base Electrical conductivity
The instructor volition test the conductivity of diverse solutions with a light seedling apparatus. The light bulb circuit is incomplete. If the circuit is completed by a solution containing a big number of ions, the light bulb will glow brightly indicating a strong ability to comport electricity as shown for HCl. If the excursion is completed by a solution containing large numbers of molecules and either no ions or few ions, the solution does not conduct or conducts very weakly as shown for acetic acid.
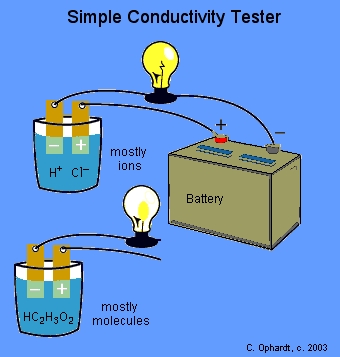
An acid or base which strongly conducts electricity contains a large number of ions and is called a strong acid or base and an acrid or base which conducts electricity just weakly contains simply a few ions and is chosen a weak acrid or base.
| Compounds | Advent of light bulb | Classification Weak or Strong | Inference of Ions or Molecules |
|---|---|---|---|
| HiiO | no light | weak | molecules |
| HCl | brilliant | strong | ions |
| HC2H3Otwo | dim | weak | molecules |
| HtwoThen4 | bright | ||
| H2CO3 | dim | ||
| NaOH | vivid | ||
| KOH | bright | ||
| NH4OH | dim |
Bond Strength
The bond strengths of acids and bases are implied by the relative amounts of molecules and ions present in solution. The bonds are represented as:
where A is a negative ion, and Grand is a positive ion
- Strong acids accept generally ions in solution, therefore the bonds property H and A together must exist weak. Strong acids easily break apart into ions.
- Weak acids exist by and large as molecules with only a few ions in solution, therefore the bonds holding H and A together must be strong. Weak acids do not readily break apart as ions but remain bonded together as molecules.
Bond Forcefulness Principle
Acids or bases with strong bonds exist predominately as molecules in solutions and are called "weak" acids or bases. Acids or bases with weak bonds easily dissociate into ions and are called "strong" acids or bases.
| Feature | Potent Acid or Base | Weak Acrid or Base of operations |
|---|---|---|
| Molecules | few | big number |
| Ions | large number | small number |
| Conductivity | strong | weak |
| Bond Strength | weak | strong |
Acids and bases behave differently in solution based on their strength. Acrid or base of operations "strength" is a measure out of how readily the molecule ionizes in water.
Introduction Again
Some acids and bases ionize quickly and nearly completely in solution; these are called stiff acids and strong bases. For example, hydrochloric acid (HCl) is a strong acid. When placed in water, most every HCl molecule splits into a H+ ion and a Cl- ion in the reaction.1
\[\ce{HCl(aq) + H2O(l) <=> H3O^{+}(aq) + Cl^{-}(aq)} \nonumber\]
For a stiff acid similar HCl, if yous place 1 mole of HCl in a liter of water, you will get roughly 1 mole of H30+ ions and ane mole of Cl- ions. In a weak acid like hydrofluoric acid (HF), not all of the HF molecules split upwards, and although in that location will exist some H+ and F- ions released, at that place will still be HF molecules in solution1. A like concept applies to bases, except the reaction is different. A stiff base similar sodium hydroxide (NaOH) volition also dissociate completely into water; if y'all put in 1 mole of NaOH into water, you volition become 1 mole of hydroxide ions.one
\[\ce{NaOH(aq) + H2O(l) <=> Na^{+}(aq) + OH^{-}(aq) + H2o(l)} \nonumber\]
The terms "stiff" and "weak" in this context do not relate to how corrosive or caustic the substance is, but only its capability to ionize in water. The ability of a substance to eat through other materials or damage peel is more of a function of the properties of that acid, besides every bit its concentration. Although, strong acids are more directly dangerous at lower concentrations a strong acid is not necessarily more dangerous than a weak i. For example, hydrofluoric acid is a weak acidane, only it is extremely dangerous and should be handled with great intendance. Hydrofluoric acid is especially unsafe because it is capable of eating through glass, as seen in the video in the links sectionV1. The percent dissociation of an acid or base is mathematically indicated past the acid ionization constant (Thoua) or the base of operations ionization constant (Thoub)1. These terms refer to the ratio of reactants to products in equilibrium when the acrid or base reacts with h2o. For acids the expression will be
Yarda = [H3O+][A-]/[HA]
where HA is the concentration of the acid at equilibrium, and A- is the concentration of its conjugate base at equilibrium and for bases the expression will be
\[K_b = \dfrac{[\ce{OH^{-}}][\ce{HB^{+}}]}{\ce{B}}\]
where B is the concentration of the base of operations at equilibrium and HB+ is the concentration of its conjugate acid at equilibrium
The stronger an acrid is, the lower the pH it volition produce in solution. pH is calculated by taking the negative logarithm of the concentration of hydronium ions. For strong acids, you can calculate the pH by simply taking the negative logarithm of its molarity as information technology completely dissociates into its conjugate base and hydronium. The same goes for strong bases, except the negative logarithm gives you the pOH as opposed to the pH. For weak acids and bases, the higher the Ka or Thoub, the more acidic or basic the solution. To find the pH for a weak acid or base of operations, you must use the M equation and a RICE table to determine the pH.
All acids have a conjugate base that forms when they react with water, and similarly, all bases have a conjugate acid that reacts when they class with water.1 Y'all can judge the relative strength of a conjugate by the \(K_a\) or \(K_b\) value of the substance because \(K_a \times K_b\) is equal to the ionization constant of h2o, One thousandw which is equal to \(1 \times 10^{-fourteen}\) at room temperature. The higher the Ka, the stronger the acid is, and the weaker its conjugate base is. Similarly, the college the Kb, the stronger the substance is every bit a base, and the more weakly acidic its cohabit acrid is.ane
Calculation of Ka
For an acid that reacts with h2o in the reaction
\[HA_{(aq)} + H_2O_{(l)} \rightleftharpoons H_3O^+_{(aq)} + A^-_{(aq)}\]
\[K_a = \dfrac{[H_3O^+][A^-]}{[HA]}\]
where each bracketed term represents the concentration of that substance in solution.
Relation of 1000due west, Mb, Thousanda
\[K_w = K_a \times K_b \nonumber\]
Partial List of Stiff Acids: Hydrochlroic acid (HCl), Nitric Acid (HNOiii), Perchloric Acrid (HClO4), Sulfuric Acid (H2SO4)
Partial List of Strong Bases: Sodium Hydroxide (NaOH), Barium Hydroxide (Ba(OH)ii), Calcium Hydroxide (Ca(OH)2), Lithium Hydroxide (LiOH) (Hydroxides of Group I and Two elements are mostly potent bases)
Fractional List of Weak Acids: Acetic Acid (CH3COOH), Carbonic Acid (HtwoCO3), Phosphoric Acrid (HiiiPO4)
Partial Listing of Weak Bases: Ammonia (NH3), Calcium Carbonate (CaCO3), Sodium Acetate (NaCHthreeCOO)
Example \(\PageIndex{ane}\)
Detect the pH of 0.five grams of HCl disolved into 100 ml of h2o:
Solution
First find moles of acid:
grams / molar mass = moles
0.5 grams / (36.5 m/mole) = 0.014 moles HCl
Then find molarity:
moles / volume = molarity
0.014 moles / 0.100 L = 0.14 M
HCl is a stiff acid and completely dissociates in water, therefore the pH volition be equal to the negative logarithm of the concentration of HCl
pH = -log(H3O+)
pH = -log(0.14) = 0.85
Example \(\PageIndex{2}\)
The Ga value for acerb acid is 1.76*x-5, and the Ka value for benzoic acid is 6.46*10-v, if two solutions are made, one from each acrid, with equal concentrations, which one will have the lower pH?
Solution
The Thousanda value is a measure of the ratio between reactants and products at equilibrium. For an acrid, the reaction volition be HA + HiiO --> A- + HiiiO+ . PH is based on the concentration of the hydronium ion (H3O+) which is a product of the reaction of acid and water. A higher Ka value means a higher ratio of reactants to products, and so the acrid with the higher Ka value will be producing more than hydronium, and therefore take a lower pH. Therefore the solution of benzoic acrid will have a lower pH.
Case \(\PageIndex{3}\)
The One thousanda value of ammonium (NH4 +) is 5.6*10-10, the Kb value of ammonia (NH3) one.8*10-5, is ammonium more strongly acidic than ammonia is basic?
Solution
The relative force of an acrid or base depends on how high its Thousanda or Kb value is, in this case, the Ka value is far lower than the Thousandb value so the ammonia is more strongly basic than ammonium is acidic.
References
- Oxtboy, Gillis, Campion, David W., H.P., Alan. "Acid-Base of operations Equilibria." Principles of Modern Chemistry. Belmont: Thomson Higher Education, 2008.
Contributors and Attributions
- Charles Ophardt, Professor Emeritus, Elmhurst College; Virtual Chembook,. Lloyd McCarthy (UCD)
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_%28Physical_and_Theoretical_Chemistry%29/Acids_and_Bases/Ionization_Constants/Acid_and_Base_Strength
0 Response to "18-2 Review and Reinforcement Determining the Strengths of Acids and Bases Answers"
Postar um comentário